CHEMISTRY WORKSHEET
Chemistry is the scientific discipline involved with compounds composed of atoms, i.e. elements, and molecules, i.e. combinations of atoms: their composition, structure, properties, behavior and the changes they undergo during a reaction with other compounds. Chemistry addresses topics such as how atoms and molecules interact via chemical bonds to form new chemical compounds. There are four types of chemical bonds: covalent bonds, in which compounds share one or more electron(s); ionic bonds, in which a compound donates one or more electrons to another compound to produce ions: cations and anions; hydrogen bonds; and Van der Waals force bonds. See glossary of chemistry.
There are four types of chemical bonds: covalent bonds, in which compounds share one or more electron(s); ionic bonds, in which a compound donates one or more electrons to another compound to produce ions: cations and anions; hydrogen bonds; and Van der Waals force bonds. See glossary of chemistry.
In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level.[4] Examples include plant chemistry (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties of the soil on the moon (astrophysics), how medications work (pharmacology), and how to collect DNA evidence at a crime scene (forensics).
The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze. The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work. Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.[5] Chemistry was preceded by its protoscience, alchemy, which is an intuitive but non-scientific approach to understanding the elements, compounds, and their interactions. It was unsuccessful in explaining the nature of matter and its transformations, but by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation between alchemy and chemistry was made by Robert Boyle in 1661: the application of the scientific method in chemistry was the crucial difference.
History of definition
The definition of chemistry has changed over time, as new discoveries and theories add to the functionality of the science. The term “chymistry”, in the view of noted scientist Robert Boyle in 1661, meant the subject of the material principles of mixed bodies. In 1663 the chemist Christopher Glaserdescribed “chymistry” as a scientific art, by which one learns to dissolve bodies, and draw from them the different substances on their composition, and how to unite them again, and exalt them to a higher perfection.
 The 1730 definition of the word “chemistry”, as used by Georg Ernst Stahl, meant the art of resolving mixed, compound, or aggregate bodies into their principles; and of composing such bodies from those principles. In 1837, Jean-Baptiste Dumas considered the word “chemistry” to refer to the science concerned with the laws and effects of molecular forces.This definition further evolved until, in 1947, it came to mean the science of substances: their structure, their properties, and the reactions that change them into other substances – a characterization accepted by Linus Pauling. More recently, in 1998, Professor Raymond Chang broadened the definition of “chemistry” to mean the study of matter and the changes it undergoes.
The 1730 definition of the word “chemistry”, as used by Georg Ernst Stahl, meant the art of resolving mixed, compound, or aggregate bodies into their principles; and of composing such bodies from those principles. In 1837, Jean-Baptiste Dumas considered the word “chemistry” to refer to the science concerned with the laws and effects of molecular forces.This definition further evolved until, in 1947, it came to mean the science of substances: their structure, their properties, and the reactions that change them into other substances – a characterization accepted by Linus Pauling. More recently, in 1998, Professor Raymond Chang broadened the definition of “chemistry” to mean the study of matter and the changes it undergoes.
English scientist John Dalton proposed the modern theory of atoms; that all substances are composed of indivisible ‘atoms’ of matter and that different atoms have varying atomic weights.
The development of the electrochemical theory of chemical combinations occurred in the early 19th century as the result of the work of two scientists in particular, J. J. Berzelius and Humphry Davy, made possible by the prior invention of the voltaic pile by Alessandro Volta. Davy discovered nine new elements including the alkali metals by extracting them from their oxides with electric current.[64]
British William Prout first proposed ordering all the elements by their atomic weight as all atoms had a weight that was an exact multiple of the atomic weight of hydrogen. J. A. R. Newlands devised an early table of elements, which was then developed into the modern periodic table of elements[65] in the 1860s by Dmitri Mendeleev and independently by several other scientists including Julius Lothar Meyer.[66][67] The inert gases, later called the noble gases were discovered by William Ramsay in collaboration with Lord Rayleigh at the end of the century, thereby filling in the basic structure of the table.
Top: Expected results: alpha particles passing through the plum pudding model of the atom undisturbed.
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated charge.
At the turn of the twentieth century the theoretical underpinnings of chemistry were finally understood due to a series of remarkable discoveries that succeeded in probing and discovering the very nature of the internal structure of atoms. 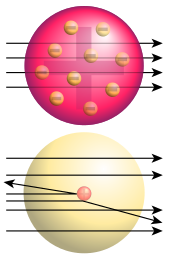
In 1897, J. J. Thomson of Cambridge University discovered the electron and soon after the French scientist Becquerel as well as the couple Pierre and Marie Curie investigated the phenomenon of radioactivity. In a series of pioneering scattering experiments Ernest Rutherford at the University of Manchester discovered the internal structure of the atom and the existence of the proton, classified and explained the different types of radioactivity and successfully transmutedthe first element by bombarding nitrogen with alpha particles.
His work on atomic structure was improved on by his students, the Danish physicist Niels Bohr and Henry Moseley. The electronic theory of chemical bonds and molecular orbitals was developed by the American scientists Linus Pauling and Gilbert N. Lewis.
The year 2011 was declared by the United Nations as the International Year of Chemistry.[68] It was an initiative of the International Union of Pure and Applied Chemistry, and of the United Nations Educational, Scientific, and Cultural Organization and involves chemical societies, academics, and institutions worldwide and relied on individual initiatives to organize local and regional activities.
Organic chemistry was developed by Justus von Liebig and others, following Friedrich Wöhler’s synthesis of urea which proved that living organisms were, in theory, reducible to chemistry.[69] Other crucial 19th century advances were; an understanding of valence bonding (Edward Frankland in 1852) and the application of thermodynamics to chemistry (J. W. Gibbs and Svante Arrhenius in the 1870s).

