WORKSHEET HERE
Lead is a chemical element with symbol Pb (from the Latin plumbum) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and has a relatively low melting point. When freshly cut, lead is bluish-white; it tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and concludes three major decay chains of heavier elements.
Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the group, lead tends to bond with itself; it can form chains, rings and polyhedral structures.
Lead is easily extracted from its ores; prehistoric people in Western Asia knew of it. Galena, a principal ore of lead, often bears silver, interest in which helped initiate widespread extraction and use of lead in ancient Rome. Lead production declined after the fall of Rome and did not reach comparable levels until the Industrial Revolution. In 2014, annual global production of lead was about ten million tonnes, over half of which was from recycling. Lead’s high density, low melting point, ductility and relative inertness to oxidation make it useful. These properties, combined with its relative abundance and low cost, resulted in its extensive use in construction, plumbing, batteries, bullets and shot, weights, solders, pewters, fusible alloys, white paints, leaded gasoline, and radiation shielding.
In the late 19th century, lead’s toxicity was recognized, and its use has since been phased out of many applications. Lead is a neurotoxin that accumulates in soft tissues and bones, damages the nervous system, and causes blood disorders. It is particularly problematic in children: even if blood levels are promptly normalized with treatment, permanent brain damage may result.
Physical properties
Atomic
A lead atom has 82 electrons, arranged in an electron configuration of [Xe]4f145d106s26p2. The combined first and second ionization energies—the total energy required to remove the two 6p electrons—is close to that of tin, lead’s upper neighbor in the carbon group. This is unusual; ionization energies generally fall going down a group, as an element’s outer electrons become more distant from the nucleus, and more shielded by smaller orbitals. The similarity of ionization energies is caused by the lanthanide contraction—the decrease in element radii from lanthanum (atomic number 57) to lutetium (71), and the relatively small radii of the elements after hafnium (72). This is due to poor shielding of the nucleus by the lanthanide 4f electrons. The combined first four ionization energies of lead exceed those of tin,[3] contrary to what periodic trends would predict. Relativistic effects, which become significant in heavier atoms, contribute to this behavior.[a] One such effect is the inert pair effect: the 6s electrons of lead become reluctant to participate in bonding, making the distance between nearest atoms in crystalline lead unusually long.[5]
Lead’s lighter carbon group congeners form stable or metastable allotropes with the tetrahedrally coordinated and covalently bonded diamond cubic structure. The energy levels of their outer s- and p-orbitals are close enough to allow mixing into four hybrid sp3 orbitals. In lead, the inert pair effect increases the separation between its s- and p-orbitals, and the gap cannot be overcome by the energy that would be released by extra bonds following hybridization.[6] Rather than having a diamond cubic structure, lead forms metallic bonds in which only the p-electrons are delocalized and shared between the Pb2+ ions. Lead consequently has a face-centered cubic structure like the similarly sized divalent metals calcium and strontium.[7][b][c]
Bulk
Pure lead has a bright silvery appearance with a hint of blue.[11] It tarnishes on contact with moist air, and takes on a dull appearance the hue of which depends on the prevailing conditions. Characteristic properties of lead include high density, malleability, and high resistance to corrosion (due to passivation).[12]
Lead’s close-packed face-centered cubic structure and high atomic weight result in a density[13] of 11.34 g/cm3, which is greater than that of common metals such as iron (7.87 g/cm3), copper (8.93 g/cm3), and zinc (7.14 g/cm3).[14] This density is the origin of the idiom to go over like a lead balloon.[15][16][d] Some rarer metals are denser: tungsten and gold are both 19.3 g/cm3, and osmium—the densest metal known—has a density of 22.59 g/cm3, almost twice that of lead.[17]
Lead is a very soft metal with a Mohs hardness of 1.5; it can be scratched with a fingernail.[18] It is quite malleable and somewhat ductile.[19][e] The bulk modulus of lead—a measure of its ease of compressibility—is 45.8 GPa. In comparison, that of aluminium is 75.2 GPa; copper 137.8 GPa; and mild steel 160–169 GPa.[20] Lead’s tensile strength, at 12–17 MPa, is low (that of aluminium is 6 times higher, copper 10 times, and mild steel 15 times higher); it can be strengthened by adding small amounts of copper or antimony.[21]
The melting point of lead—at 327.5 °C (621.5 °F)[22]—is very low compared to most metals.[13][f] Its boiling point of 1749 °C (3180 °F)[22] is the lowest among the carbon group elements. The electrical resistivity of lead at 20 °C is 192 nanoohm–meters, almost an order of magnitude higher than those of other industrial metals (copper at 15.43 nΩ·m; gold 20.51 nΩ·m; and aluminium at 24.15 nΩ·m).[24] Lead is a superconductor at temperatures lower than 7.19 K;[25] this is the highest critical temperature of all type-I superconductors and the third highest of the elemental superconductors.[26]
Isotopic abundances vary greatly by sample
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight(Ar, standard) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Isotopes[edit]
Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208,[27] and traces of five short-lived radioisotopes.[28] The high number of isotopes is consistent with lead’s atomic number being even.[g] Lead has a magic number of protons (82), for which the nuclear shell model accurately predicts an especially stable nucleus. Lead-208 has 126 neutrons, another magic number, which may explain why lead-208 is extraordinarily stable.[29]
With its high atomic number, lead is the heaviest element whose natural isotopes are regarded as stable; lead-208 is the heaviest stable nucleus. This title was formerly held by bismuth, with an atomic number of 83, until its only primordial isotope, bismuth-209, was found in 2003 to decay very slowly.[h] The four stable isotopes of lead could theoretically undergo alpha decay to isotopes of mercury with a release of energy, but this has not been observed for any of them; their predicted half-lives range from 1035 to 10189 years.[32]
Three of the stable isotopes are found in three of the four major decay chains: lead-206, lead-207, and lead-208 are the final decay products of uranium-238, uranium-235, and thorium-232, respectively. These decay chains are called the uranium series, the actinium series, and the thorium series. Their isotopic concentration in a natural rock sample depends greatly on the presence of these three parent uranium and thorium isotopes. For example, the relative abundance of lead-208 can range from 52% in normal samples to 90% in thorium ores;[33] for this reason, the standard atomic weight of lead is given to only one decimal place.[34] As time passes, the ratio of lead-206 and lead-207 to lead-204 increases, since the former two are supplemented by radioactive decay of heavier elements while the latter is not; this allows for lead–lead dating. As uranium decays into lead, their relative amounts change; this is the basis for uranium–lead dating.[35]

The Holsinger meteorite, the largest piece of the Canyon Diablo meteorite. Uranium–lead dating and lead–lead dating on this meteorite allowed refinement of the age of the Earth to 4.55 billion ± 70 million years.
Apart from the stable isotopes, which make up almost all lead that exists naturally, there are trace quantities of a few radioactive isotopes. One of them is lead-210; although it has a half-life of only 22.3 years,[27] small quantities occur in nature because lead-210 is produced by a long decay series that starts with uranium-238 (which has been present for billions of years on Earth). Lead-211, -212, and -214 are present in the decay chains of uranium-235, thorium-232, and uranium-238, respectively, so traces of all three of these lead isotopes are found naturally. Minute traces of lead-209 arise from the very rare cluster decay of radium-223, one of the daughter products of natural uranium-235. Lead-210 is particularly useful for helping to identify the ages of samples by measuring its ratio to lead-206 (both isotopes are present in a single decay chain).[36]
In total, 43 lead isotopes have been synthesized, with mass numbers 178–220.[27] Lead-205 is the most stable radioisotope, with a half-life of around 1.5×107 years.[i] The second-most stable is lead-202, which has a half-life of about 53,000 years, longer than any of the natural trace radioisotopes.[27]
Chemistry[edit]

Flame test: lead colors flame pale blue
Bulk lead exposed to moist air forms a protective layer of varying composition. Lead carbonate is a common constituent;[38][39][40] the sulfate or chloride may also be present in urban or maritime settings.[41] This layer makes bulk lead effectively chemically inert in the air.[41] Finely powdered lead, as with many metals, is pyrophoric,[42] and burns with a bluish-white flame.[43]
Fluorine reacts with lead at room temperature, forming lead(II) fluoride. The reaction with chlorine is similar but requires heating as the resulting chloride layer diminishes the reactivity of the elements.[41] Molten lead reacts with the chalcogens to give lead(II) chalcogenides.[44]
Lead metal resists sulfuric and phosphoric acid but not hydrochloric or nitric acid; the outcome depends on insolubility and subsequent passivation of the product salt.[45] Organic acids, such as acetic acid, dissolve lead in the presence of oxygen.[41] Concentrated alkalis will dissolve lead and form plumbites.[46]
Inorganic compounds[edit]
Lead shows two main oxidation states: +4 and +2. The tetravalent state is common for the carbon group. The divalent state is rare for carbon and silicon, minor for germanium, important (but not prevailing) for tin, and is the more important for lead.[41] This is attributable to relativistic effects, specifically the inert pair effect, which manifests itself when there is a large difference in electronegativity between lead and oxide, halide, or nitride anions, leading to a significant partial positive charge on lead. The result is a stronger contraction of the lead 6s orbital than is the case for the 6p orbital, making it rather inert in ionic compounds. This is less applicable to compounds in which lead forms covalent bonds with elements of similar electronegativity such as carbon in organolead compounds. In these, the 6s and 6p orbitals remain similarly sized and sp3 hybridization is still energetically favorable. Lead, like carbon, is predominantly tetravalent in such compounds.[47]
There is a relatively large difference in the electronegativity of lead(II) at 1.87 and lead(IV) at 2.33. This difference marks the reversal in the trend of increasing stability of the +4 oxidation state going down carbon group; tin, by comparison, has values of 1.80 in the +2 oxidation state and 1.96 in the +4 state.[48]
Lead(II)[edit]
Lead(II) compounds are characteristic of the inorganic chemistry of lead. Even strong oxidizing agents like fluorine and chlorine react with lead to give only PbF2 and PbCl2.[41] Lead(II) ions are usually colorless in solution,[49] and partially hydrolyze to form Pb(OH)+ and finally Pb4(OH)4 (in which the hydroxyl ions act as bridging ligands),[50][51] but are not reducing agents as tin(II) ions are. Techniques for identifying the presence of the Pb2+ ion in water generally rely on the precipitation of lead(II) chloride using dilute hydrochloric acid. As the chloride salt is somewhat soluble in water, the precipitation of lead(II) sulfide, by bubbling hydrogen sulfide through the solution, is then attempted.[52]
Lead monoxide exists in two polymorphs, red α-PbO and yellow β-PbO, the latter being stable only above around 488 °C. It is the most commonly used compound of lead.[53] Its lead(II) hydroxide counterpart can only exist in solution; it is known to form plumbite anions.[54] Lead commonly reacts with heavier chalcogens. Lead sulfide is a semiconductor, a photoconductor, and an extremely sensitive infrared radiation detector. The other two chalcogenides, lead selenide and lead telluride, are likewise photoconducting. They are unusual in that their color becomes lighter going down the group.[55]

Lead and oxygen in a tetragonal unit cell of lead(II,IV) oxide
Lead dihalides are well-characterized; this includes the diastatide,[56] and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the gravimetricdetermination of fluorine. The difluoride was the first solid ionically conducting compound to be discovered (in 1834, by Michael Faraday).[57] The other dihalides decompose on exposure to ultraviolet or visible light, especially the diiodide.[58] Many lead pseudohalides are known.[55] Lead(II) forms an extensive variety of halide coordination complexes, such as [PbCl4]2−, [PbCl6]4−, and the [Pb2Cl9]n5n− chain anion.[58]
Lead(II) sulfate is insoluble in water, like the sulfates of other heavy divalent cations. Lead(II) nitrate and lead(II) acetate are very soluble, and this is exploited in the synthesis of other lead compounds.[59]
Lead(IV)
Few inorganic lead(IV) compounds are known, and these exist only in highly acidic solutions.[60] Lead(II) oxide gives a mixed oxide on further oxidation, Pb3O4. It is described as lead(II,IV) oxide, or structurally 2PbO·PbO2, and is the best-known mixed valence lead compound. Lead dioxide is a strong oxidizing agent, capable of oxidizing hydrochloric acid to chlorine gas. This is because the expected PbCl4 that would be produced is unstable and spontaneously decomposes to PbCl2 and Cl2. Analogously to lead monoxide, lead dioxide is capable of forming plumbate anions. Lead disulfide[61] and lead diselenide[62] are only stable at high pressures. Lead tetrafluoride, a yellow crystalline powder, is stable, but less so than the difluoride. Lead tetrachloride (a yellow oil) decomposes at room temperature, lead tetrabromide is less stable still, and the existence of lead tetraiodide is questionable.[63]
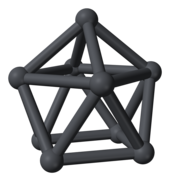
The capped square antiprismatic anion [Pb9]4−from [K(18-crown-6)]2K2Pb9·(en)1.5[64]
Other oxidation states[edit]
Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable as both the lead(III) ion and the larger complexes containing it are radicals.[65][66][67] The same applies for lead(I), which can be found in such species.[68]
Numerous mixed lead(II,IV) oxides are known. When PbO2 is heated in air, it becomes Pb12O19 at 293 °C, Pb12O17 at 351 °C, Pb3O4 at 374 °C, and finally PbO at 605 °C. A further sesquioxide Pb2O3 can be obtained at high pressure, along with several non-stoichiometric phases. Many of them show defective fluorite structures in which some oxygen atoms are replaced by vacancies: PbO can be considered as having such a structure, with every alternate layer of oxygen atoms absent.[69]
Negative oxidation states can occur as Zintl phases, as either free lead anions, as in Ba2Pb, with lead formally being lead(−IV),[70] or in oxygen-sensitive ring-shaped or polyhedral cluster ions such as the trigonal bipyramidal Pb52− ion, where two lead atoms are lead(−I) and three are lead(0).[71] In such anions, each atom is at a polyhedral vertex and contributes two electrons to each covalent bond along an edge from their sp3 hybrid orbitals, the other two being an external lone pair.[50] They may be made in liquid ammonia via the reduction of lead by sodium.[72]

Structure of a tetraethyllead molecule:
Carbon
Hydrogen
Lead
Organolead[edit]
Lead can form multiply bonded chains, a property it shares with its lighter homolog, carbon. Its capacity to do so is much less because the Pb–Pb bond energy is over three and a half times lower than that of the C–C bond.[44] With itself lead can build metal–metal bonds of an order up to three.[73] With carbon, lead forms organolead compounds similar to, but generally less stable than, typical organic compounds[74] (due to the Pb–C bond being rather weak).[50] This makes the organometallic chemistry of lead far less wide-ranging than that of tin.[75] It predominantly forms organolead(IV) compounds, even when starting with inorganic lead(II) reactants; very few organolead(II) compounds are known. The most well-characterized exceptions are Pb[CH(SiMe3)2]2 and Pb(η5-C5H5)2.[75]
The lead analog of the simplest organic compound, methane, is plumbane. Plumbane may be obtained in a reaction between metallic lead and atomic hydrogen.[76] Two simple derivatives, tetramethyllead and tetraethyllead, are the best-known organolead compounds. These compounds are relatively stable: tetraethyllead only starts to decompose if heated[77] or if exposed to sunlight or ultraviolet light.[78] (Tetraphenyllead is even more thermally stable, decomposing at 270 °C.[75]) With sodium metal, lead readily forms an equimolar alloy that reacts with alkyl halides to form organometallic compounds such as tetraethyllead.[79] The oxidizing nature of many organolead compounds is usefully exploited: lead tetraacetate is an important laboratory reagent for oxidation in organic chemistry[80] and tetraethyllead was once produced in larger quantities than any other organometallic compound.[75] Other organolead compounds are less chemically stable.[74] For many organic compounds, a lead analog does not exist.[76]
Origin and occurrence[edit]
| Atomic number |
Element | Relative amount |
|---|---|---|
| 42 | Molybdenum | 0.798 |
| 46 | Palladium | 0.440 |
| 50 | Tin | 1.146 |
| 78 | Platinum | 0.417 |
| 80 | Mercury | 0.127 |
| 82 | Lead | 1 |
| 90 | Thorium | 0.011 |
| 92 | Uranium | 0.003 |
In space[edit]
Lead’s per-particle abundance in the Solar System is 0.121 ppb (parts per billion).[81][j] This figure is two and a half times higher than that of platinum, eight times more than mercury, and seventeen times more than gold.[81] The amount of lead in the universe is slowly increasing[82] as most heavier atoms (all of which are unstable) gradually decay to lead.[83] The abundance of lead in the Solar System since its formation 4.5 billion years ago has increased by about 0.75%.[84] The solar system abundances table shows that lead, despite its relatively high atomic number, is more prevalent than most other elements with atomic numbers greater than 40.[81]
Primordial lead—which comprises the isotopes lead-204, lead-206, lead-207, and lead-208—was mostly created as a result of repetitive neutron capture processes occurring in stars. The two main modes of capture are the s- and r-processes.[85]
In the s-process (s is for “slow”), captures are separated by years or decades, allowing less stable nuclei to undergo beta decay. A stable thallium-203 nucleus can capture a neutron and become thallium-204; this undergoes beta decay to give stable lead-204; on capturing another neutron, it becomes lead-205, which has a half-life of around 15 million years. Further captures result in lead-206, lead-207, and lead-208. On capturing another neutron, lead-208 becomes lead-209, which quickly decays into bismuth-209. On capturing another neutron, bismuth-209 becomes bismuth-210, and this beta decays to polonium-210, which alpha decays to lead-206. The cycle hence ends at lead-206, lead-207, lead-208, and bismuth-209.[86]

Chart of the final part of the s-process, from mercury to polonium. Red lines and circles represent neutron captures; blue arrows represent beta decays; the green arrow represents an alpha decay; cyan arrows represent electron captures.
In the r-process (r is for “rapid”), captures happen faster than nuclei can decay. This occurs in environments with a high neutron density, such as a supernova or the merger of two neutron stars. The neutron flux involved may be on the order of 1022 neutrons per square centimeter per second.[87] The r-process does not form as much lead as the s-process. It tends to stop once neutron-rich nuclei reach 126 neutrons. At this point, the neutrons are arranged in complete shells in the atomic nucleus, and it becomes harder to energetically accommodate more of them. When the neutron flux subsides, these nuclei beta decay into stable isotopes of osmium, iridium, and platinum.[88]
On Earth[edit]
Lead is classified as a chalcophile under the Goldschmidt classification, meaning it is generally found combined with sulfur.[89] It rarely occurs in its native, metallic form.[90] Many lead minerals are relatively light and, over the course of the Earth’s history, have remained in the crust instead of sinking deeper into the Earth’s interior. This accounts for lead’s relatively high crustal abundance of 14 ppm; it is the 38th most abundant element in the crust.[91][k]
The main lead-bearing mineral is galena (PbS), which is mostly found with zinc ores.[93] Most other lead minerals are related to galena in some way; boulangerite, Pb5Sb4S11, is a mixed sulfide derived from galena; anglesite, PbSO4, is a product of galena oxidation; and cerussite or white lead ore, PbCO3, is a decomposition product of galena. Arsenic, tin, antimony, silver, gold, copper, and bismuth are common impurities in lead minerals.[93]

Lead is a fairly common element in the Earth’s crust for its high atomic number (82). Most elements of atomic number greater than 40 are less abundant.
World lead resources exceed 2 billion tons. Significant deposits are located in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, and the United States. Global reserves—resources that are economically feasible to extract—totaled 88 million tons in 2016, of which Australia had 35 million, China 17 million, and Russia 6.4 million.[94]
Typical background concentrations of lead do not exceed 0.1 μg/m3 in the atmosphere; 100 mg/kg in soil; and 5 μg/L in freshwater and seawater.[95]
Etymology[edit]
The modern English word “lead” is of Germanic origin; it comes from the Middle English leed and Old English lēad (with the macron above the “e” signifying that the vowel sound of that letter is long).[96] The Old English word is derived from the hypothetical reconstructed Proto-Germanic *lauda- (“lead”).[97] According to linguistic theory, this word bore descendants in multiple Germanic languages of exactly the same meaning.[97]
The origin of the Proto-Germanic *lauda- is not agreed in the linguistic community. One hypothesis suggests it is derived from Proto-Indo-European *lAudh- (“lead”; capitalization of the vowel is equivalent to the macron).[98] Another hypothesis suggests it is borrowed from Proto-Celtic *ɸloud-io- (“lead”). This word is related to the Latin plumbum, which gave the element its chemical symbol Pb. The word *ɸloud-io- is thought to be the origin of Proto-Germanic *bliwa- (which also means “lead”), from which stemmed the German Blei.[99]
The name of the chemical element is not related to the verb of the same spelling, which is derived from Proto-Germanic *laidijan- (“to lead”).[100]
History[edit]
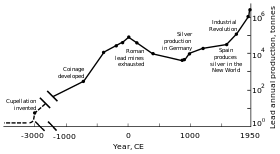
World lead production peaking in the Romanperiod and the Industrial Revolution.[101]
Prehistory and early history[edit]
Metallic lead beads dating back to 7000–6500 BCE have been found in Asia Minor and may represent the first example of metal smelting.[102] At that time lead had few (if any) applications due to its softness and dull appearance.[102] The major reason for the spread of lead production was its association with silver, which may be obtained by burning galena (a common lead mineral).[103] The Ancient Egyptians were the first to use lead minerals in cosmetics, an application that spread to Ancient Greece and beyond;[104] the Egyptians may have used lead for sinkers in fishing nets, glazes, glasses, enamels, and for ornaments.[103] Various civilizations of the Fertile Crescent used lead as a writing material, as currency, and for construction.[103] Lead was used in the Ancient Chinese royal court as a stimulant,[103] as currency,[105] and as a contraceptive;[106] the Indus Valley civilization and the Mesoamericans[103] used it for making amulets; and the eastern and southern African peoples used lead in wire drawing.[107]
Classical era[edit]
Because silver was extensively used as a decorative material and an exchange medium, lead deposits came to be worked in Asia Minor since 3000 BCE; later, lead deposits were developed in the Aegean and Laurion. These three regions collectively dominated production of mined lead until c. 1200 BCE.[108] Since 2000 BCE, the Phoenicians worked deposits in the Iberian peninsula; by 1600 BCE, lead mining existed in Cyprus, Greece, and Sardinia.[109]

Ancient Greek lead sling bullets with a winged thunderbolt molded on one side and the inscription “ΔΕΞΑΙ” (“take that” or “catch”) on the other side[110]
Rome’s territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the classical era, with an estimated annual output peaking at 80,000 tonnes. Like their predecessors, the Romans obtained lead mostly as a by-product of silver smelting.[101][111] Lead mining occurred in Central Europe, Britain, the Balkans, Greece, Anatolia, and Hispania, the latter accounting for 40% of world production.[101]
Lead was used for making water pipes in the Roman Empire; the Latin word for the metal, plumbum, is the origin of the English word “plumbing“. Its ease of working and resistance to corrosion[112] ensured its widespread use in other applications including pharmaceuticals, roofing, currency, and warfare.[113][114][115] Writers of the time, such as Cato the Elder, Columella, and Pliny the Elder, recommended lead (or lead-coated) vessels for the preparation of sweeteners and preservatives added to wine and food. The lead conferred an agreeable taste due to the formation of “sugar of lead” (lead(II) acetate), whereas copper or bronze vessels could impart a bitter flavor through verdigris formation.[116]
Heinz Eschnauer and Markus Stoeppler
“Wine—An enological specimen bank”, 1992[117]
The Roman author Vitruvius reported the health dangers of lead[118] and modern writers have suggested that lead poisoning played a major role in the decline of the Roman Empire.[119][120][l] Other researchers have criticized such claims, pointing out, for instance, that not all abdominal pain is caused by lead poisoning.[122][123] According to archaeological research, Roman lead pipes increased lead levels in tap water but such an effect was “unlikely to have been truly harmful”.[124][125] When lead poisoning did occur, victims were called “saturnine”, dark and cynical, after the ghoulish father of the gods, Saturn. By association, lead was considered the father of all metals.[126] Its status in Roman society was low as it was readily available[127] and cheap.[128]

Roman lead pipes[m]
Confusion with tin and antimony[edit]
During the classical era (and even up to the 17th century), tin was often not distinguished from lead: Romans called lead plumbum nigrum (“black lead”), and tin plumbum candidum (“bright lead”). The association of lead and tin can be seen in other languages: the word olovo in Czech translates to “lead”, but in Russian the cognate олово(olovo) means “tin”.[129] To add to the confusion, lead bore a close relation to antimony: both elements commonly occur as sulfides (galena and stibnite), often together. Pliny incorrectly wrote that stibnite would give lead on heating, instead of antimony.[130] In countries such as Turkey and India, the originally Persian name surma came to refer to either antimony sulfide or lead sulfide,[131] and in some languages, such as Russian, gave its name to antimony (сурьма).[132]
Middle Ages and the Renaissance[edit]
Lead mining in Western Europe declined after the fall of the Western Roman Empire, with Arabian Iberia being the only region having a significant output.[133][134] The largest production of lead occurred in South and East Asia, especially China and India, where lead mining grew strongly.[134]

Elizabeth I of England was commonly depicted with a whitened face. Lead in face whiteners is thought to have contributed to her death.[135]
In Europe, lead production only began to revive in the 11th and 12th centuries, when it was again used for roofing and piping. Starting in the 13th century, lead was used to create stained glass.[136] In the European and Arabian traditions of alchemy, lead (symbol ![]() in the European tradition)[137] was considered an impure base metal which, by the separation, purification and balancing of its constituent essences, could be transformed to pure and incorruptible gold.[138] During the period, lead was used increasingly for adulterating wine. The use of such wine was forbidden for use in Christian rites by a papal bull in 1498, but it continued to be imbibed and resulted in mass poisonings up to the late 18th century.[133][139] Lead was a key material in parts of the printing press, which was invented around 1440; lead dust was commonly inhaled by print workers, causing lead poisoning.[140] Firearms were invented at around the same time, and lead, despite being more expensive than iron, became the chief material for making bullets. It was less damaging to iron gun barrels, had a higher density (which allowed for better retention of velocity), and its lower melting point made the production of bullets easier as they could be made using a wood fire.[141] Lead, in the form of Venetian ceruse, was extensively used in cosmetics by Western European aristocracy as whitened faces were regarded as a sign of modesty.[142][143] This practice later expanded to white wigs and eyeliners, and only faded out with the French Revolution in the late 18th century. A similar fashion appeared in Japan in the 18th century with the emergence of the geishas, a practice that continued long into the 20th century. The white faces of women “came to represent their feminine virtue as Japanese women”,[144] with lead commonly used in the whitener.[145]
in the European tradition)[137] was considered an impure base metal which, by the separation, purification and balancing of its constituent essences, could be transformed to pure and incorruptible gold.[138] During the period, lead was used increasingly for adulterating wine. The use of such wine was forbidden for use in Christian rites by a papal bull in 1498, but it continued to be imbibed and resulted in mass poisonings up to the late 18th century.[133][139] Lead was a key material in parts of the printing press, which was invented around 1440; lead dust was commonly inhaled by print workers, causing lead poisoning.[140] Firearms were invented at around the same time, and lead, despite being more expensive than iron, became the chief material for making bullets. It was less damaging to iron gun barrels, had a higher density (which allowed for better retention of velocity), and its lower melting point made the production of bullets easier as they could be made using a wood fire.[141] Lead, in the form of Venetian ceruse, was extensively used in cosmetics by Western European aristocracy as whitened faces were regarded as a sign of modesty.[142][143] This practice later expanded to white wigs and eyeliners, and only faded out with the French Revolution in the late 18th century. A similar fashion appeared in Japan in the 18th century with the emergence of the geishas, a practice that continued long into the 20th century. The white faces of women “came to represent their feminine virtue as Japanese women”,[144] with lead commonly used in the whitener.[145]
Outside Europe and Asia[edit]
In the New World, lead was produced soon after the arrival of European settlers. The earliest recorded lead production dates to 1621 in the English Colony of Virginia, fourteen years after its foundation.[146] In Australia, the first mine opened by colonists on the continent was a lead mine, in 1841.[147] In Africa, lead mining and smelting were known in the Benue Trough[148] and the lower Congo Basin, where lead was used for trade with Europeans, and as a currency by the 17th century,[149] well before the scramble for Africa.

Lead mining in the upper Mississippi River region in the United States in 1865
Industrial Revolution[edit]
In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the Industrial Revolution. This was the first time during which production rates exceeded those of Rome.[101] Britain was the leading producer, losing this status by the mid-19th century with the depletion of its mines and the development of lead mining in Germany, Spain, and the United States.[150] By 1900, the United States was the leader in global lead production, and other non-European nations—Canada, Mexico, and Australia—had begun significant production; production outside Europe exceeded that within.[151] A great share of the demand for lead came from plumbing and painting—lead paints were in regular use.[152] At this time, more (working class) people were exposed to the metal and lead poisoning cases escalated. This led to research into the effects of lead intake. Lead was proven to be more dangerous in its fume form than as a solid metal. Lead poisoning and gout were linked; British physician Alfred Baring Garrod noted a third of his gout patients were plumbers and painters. The effects of chronic ingestion of lead, including mental disorders, were also studied in the 19th century. The first laws aimed at decreasing lead poisoning in factories were enacted during the 1870s and 1880s in the United Kingdom.[152]

Promotional poster for Dutch Boy lead paint, United States, 1912
Modern era[edit]
Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported.[153] The last major human exposure to lead was the addition of tetraethyllead to gasoline as an antiknock agent, a practice that originated in the United States in 1921. It was phased out in the United States and the European Union by 2000.[152] Most European countries banned lead paint—commonly used because of its opacity and water resistance[154]—for interiors by 1930.[155]
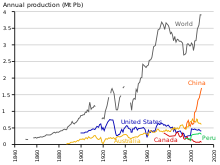
In the 1970s, the United States and Western European countries introduced legislation to reduce lead air pollution.[156][157] The impact was significant: while a study conducted by the Centers for Disease Control and Prevention in the United States in 1976–1980 showed that 77.8% of population had elevated blood lead levels, in 1991–1994, a study by the same institute showed the share of people with such high levels dropped to 2.2%.[158] The main product made of lead by the end of the 20th century was the lead–acid battery,[159]which posed no direct threat to humans. From 1960 to 1990, lead output in the Western Bloc grew by a third.[160] The share of the world’s lead production by the Eastern Blocincreased from 10% to 30%, from 1950 to 1990, with the Soviet Union being the world’s largest producer during the mid-1970s and the 1980s, and China starting major lead production in the late 20th century.[161] Unlike the European communist countries, China was largely unindustrialized by the mid-20th century; in 2004, China surpassed Australia as the largest producer of lead.[162] As was the case during European industrialization, lead has had a negative effect on health in China.[163]
Production[edit]
Production of lead is increasing worldwide due to its use in lead–acid batteries.[164] There are two major categories of production: primary from mined ores, and secondary from scrap. In 2014, 4.58 million metric tons came from primary production and 5.64 million from secondary production. The top three producers of mined lead concentrate in that year were China, Australia, and the United States. The top three producers of refined lead were China, the United States, and South Korea.[165] According to the International Resource Panel‘s Metal Stocks in Society report of 2010, the total amount of lead in use, stockpiled, discarded or dissipated into the environment, on a global basis, is 8 kg per capita. Much of this is in more developed countries (20–150 kg per capita) rather than less developed ones (1–4 kg per capita).[166]
Production processes for primary and secondary lead are similar. Some primary production plants now supplement their operations with scrap lead, and this trend is likely to increase in the future. Given adequate techniques, secondary lead is indistinguishable from primary lead. Scrap lead from the building trade is usually fairly clean and is re-melted without the need for smelting, though refining is sometimes needed. Secondary lead production is therefore cheaper, in terms of energy requirements, than is primary production, often by 50% or more.[167]